Battery Blog
Discussion on the Apple Watch Metal Can Battery: A Novel Design for Portable and Wearable Electronics
Wearables are one of the most constrained formfactors for consumer electronics. Manufactures are trying to incorporate advanced features, high levels of processing and energy storage into the smallest size and lightest weight possible. The wearable must be able to tolerate frequent charging and operate safely over time, even when subjected to changing temperatures, vibration, and impact. From our product level teardowns, TechInsights has analyzed various wearable products and pouch cells are the typical battery formfactor of choice. One exception is the Apple Watch. In 2019, the Apple Watch Series 5 line was released which consisted of a 40mm and 44mm version. In the smaller version, we found, for the first time a Metal Can formfactor. We have decided to take a deeper look into the Metal can approach with our analysis of the Apple watch series 7 (41 mm) to characterize the metal can battery with the intention of answering why the metal can is used instead of the soft pouch.
Additional detail and analysis of this battery can be found in the report "Desay's A2663 Metal Can Battery (Apple Watch Series 7 - 41mm) Essentials" which is part of the TechInsights Rechargeable Battery Essentials subscription
Review of Battery Form-factors:
The basic configuration of almost all lithium-ion batteries consists of four main parts: 1) the cathode material (usually LiCoO2 for consumer electronics), which is coated onto an aluminum foil, 2) layered graphite as the anode material, which is coated onto a copper foil, 3) an ionically conducting electrolyte usually liquid type which is made from a solution of lithium salts in organic solvents typically carbonates and 4) an insulating separator made with layers composed of polyethylene and polypropylene which is placed between the anode and cathode to avoid shorting. Based on these parts, there are four main configurations (formfactors) of lithium-ion batteries that can be found in the current market, i.e., cylindrical, prismatic pouch and button form factor, as shown in the following image.
![Figure 1: The most common lithium-ion cell types [1]](https://www.techinsights.com/sites/default/files/2022-10/Fig1_0.jpg)
Figure 1: The most common lithium-ion cell types [1]
The popularity of pouch cells
Pouch cells are the most common formfactor used in consumer electronics because they can be customized to varied sizes to maximize the use of available space in the device and reach 90%-95% packaging efficiency.
In pouch design, the stacked layers of anode, cathode and separators are encased in a soft pouch. The battery pouch is usually made up of aluminum foil laminated with organic polymer on both sides. The sealed multilayer laminate sheet of the battery pouch prevents the electrode assembly of the lithium battery from reacting with the external moisture, oxygen, and other contaminants. The sealed multilayer sheet also contains or mitigates any leakage of the electrolyte, avoiding damage to other components of the portable electronic device.
Pouch Cells and Safety
In many devices, a minimum threshold distance is established for the pouch. The minimum threshold distance is how close an edge of the pouch (or an edge of the seal, in some embodiments) to the components around it. If the flap of the pouch has a width below the minimum threshold distance, the pouch may be susceptible to tearing or breaking, and/or the seal may be susceptible to failing, especially if it is subject to significant shock or encounters expansion during cycling.
Introducing the Metal Can
As mentioned earlier, we first encountered a metal can battery in the Apple watch in 2019. Further investigation showed that Apple filed for a patent for a Metal Can Battery the same year with the Patent still pending at time of this blog. The patent describes how it is “an improved battery for reducing space between the battery and electronic components” The patent details how to optimize the available space in the electronic device without the need for spacing between the battery casing and other components in the electronic device. In some designs, the metal housing can be connected to a common ground to allow other components to contact the battery casing without causing a short circuit. Additionally, the metal casing can be used as a structural element in the electronic device. For example, brackets can be attached to the metal casing, or a flange between two housing pieces can be used as an attachment point.
Reverse Engineering the Apple watch series 7 (41 mm) and its metal can battery
At TechInsights, we opened an Apple watch series 7 (41 mm) to characterize its metal can battery. Figure 2 shows the inside of the device along with its battery after removal of the display.

Figure 2: Teardown images of Apple Smartwatch Series 7 (41 mm) after removing the display.
Table 1 compares the Apple watch series 7 (41 mm) battery with the larger version (both batteries were made by Desay Group). The results reveal that the metal can design offers a 10% decrease in the footprint area without compromising the areal capacity (capacity per area). Its energy density (energy per volume) however, is ~19% less than found in the larger watch. In Apple's smart watch Series 7 (41 mm), the height-to-width ratio of the battery is 0.193, which is significantly greater than the 45 mm version. Looking at the real product we confirmed that the metal casing is connected to a common ground to allow other components to contact the battery housing without causing a short circuit.
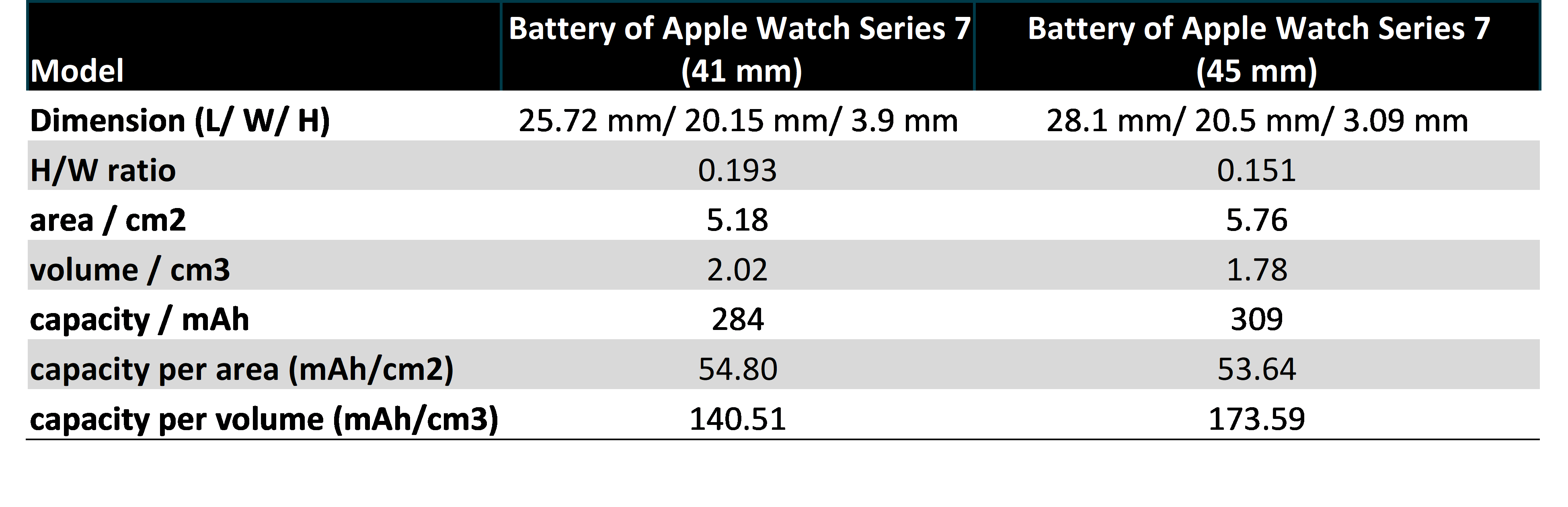
Table 1: A comparison between the batteries of Battery of Apple Watch Series 7 (41 mm) and (45 mm)
Battery Characterization
To analyze the battery further, the Apple watch series 7’s (41 mm) battery pack was removed and tested using Differential Capacity Analysis (DCA) at C/20. To compare the results with those of its larger siblings, we normalized dq/dV values by the battery’s capacity (dq/dV divided by battery’s capacity). As shown in Figure 3, both batteries share similar chemistry based on lithium cobalt oxide at the cathode and graphite at the anode.
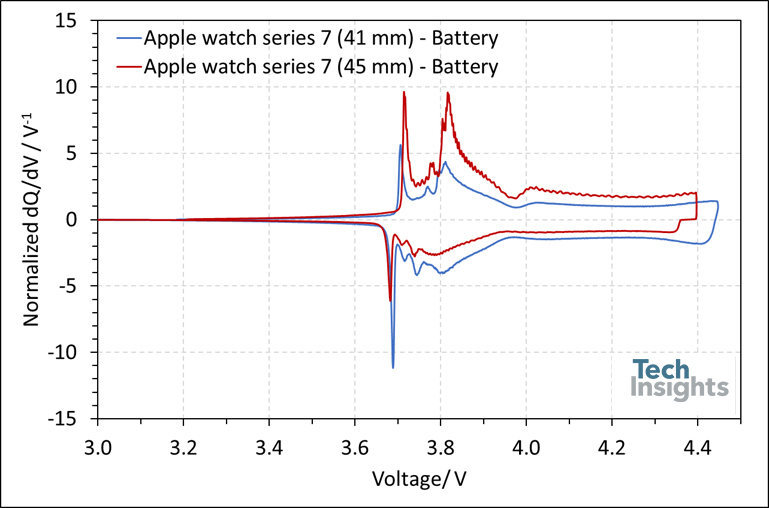
Figure 3: The differential capacity curve of the battery of Apple watch series 7 (41 mm) in comparison with the larger size.
The impedance and internal resistance of the Apple watch series 7’s (41 mm) battery were analyzed using electrochemical impedance spectroscopy (EIS) at different states of charge (SOC). EIS measurements were performed for a frequency range of 3 kHz to 50 mHz by applying a sinusoidal signal of 5 mV amplitude. The respective results are given in Figure 4 in the form of a Nyquist plot. A comparison of the different spectra reveals that they share a similar trend. Generally, each spectrum consists of two semi-circles at high-to-medium frequencies followed by a 45◦ line in the low-frequency region. The interception of the real and imaginary axis shows the overall ohmic resistance, equal to 110 mΩ. The first semi-circle represents the solid electrolyte interphase of the battery, while the second semi-circle represents the electrochemical reactions at anode and cathode. The 45◦ line corresponds to the diffusion of lithium ions. The sum of diameters of each semi-circle represents the resistances against electrochemical phenomena. For the fully discharged battery, this value is found to be ~ 0.4 Ω; as the battery charges, the resistance against the charge drops by 35%.
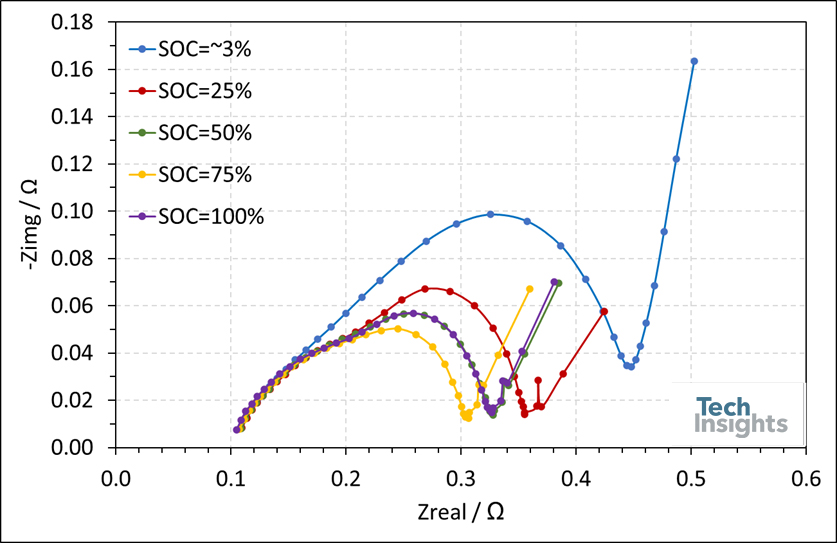
Figure 4: Nyquist plots of an Apple watch series 7’s (41 mm) battery at states of charge of 3%, 25%, 50%, 75% and 100%.
Usually, batteries with smaller capacities feature higher internal resistance. However, in the case of the Apple watch series 7’s batteries, the smaller version features similar impedance compared to its larger counterparts. (Figure 5).
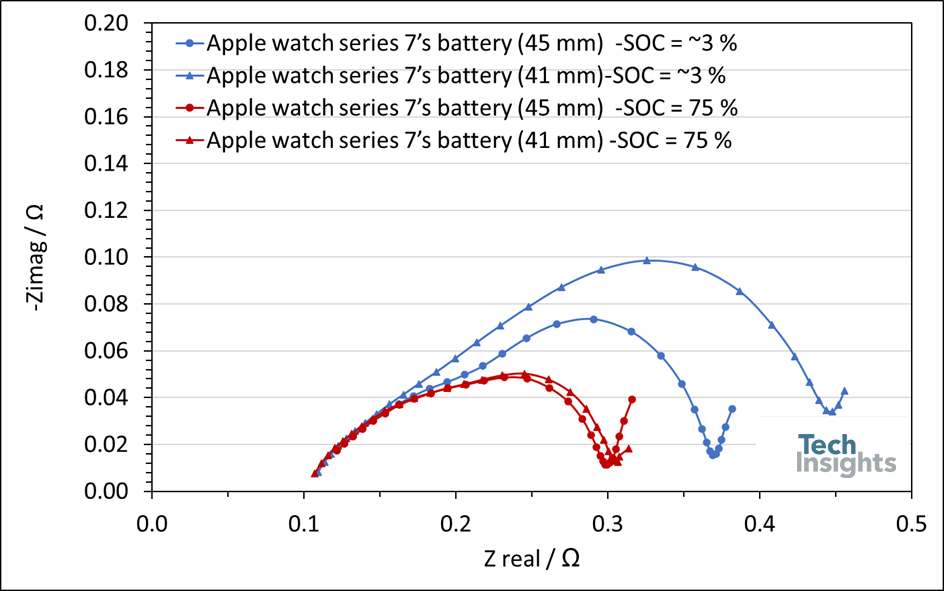
Figure 5: Nyquist plots of an Apple Watch Series 7’s batteries (41 and 44 mm) at SOC of 3% and 75%.
Battery Structural and Materials Analysis
Looking deeper into the battery design and chemistry, we disassembled the battery to understand the structure of the cells inside the can and the number of stacked layers. Figure 6 shows the battery after tearing down the metal can. The anode current collector is welded to the nickel tab (anode electrode), and the nickel tab is welded to the metal casing resulting in common ground for the entire can. The cathode current collector is connected to an aluminum tab. However, a plastic seal protects the cathode tab from contacting the metal which can result in a short circuit, as shown in the image. To prevent any possible direct contact between the jellyroll and the tabs, a polymer plate is placed inside the metal pouch, as shown in the image.
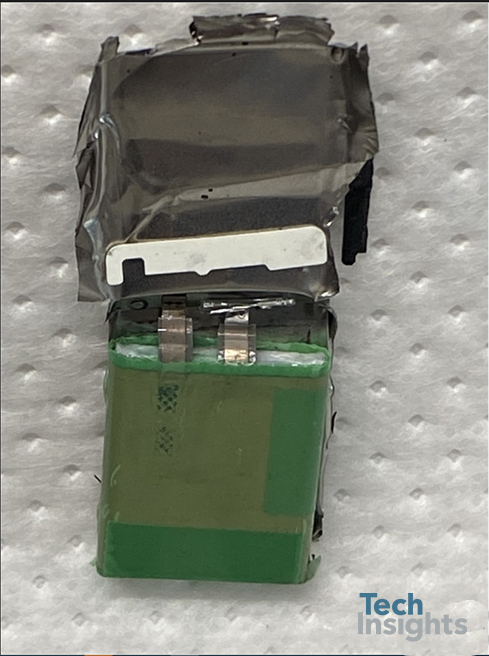
Figure 6: Teardown image of Apple watch series 7 (41 mm)’s battery after opening the metal pouch.
Electrode Structural Analysis
Figure 7 illustrates an SEM (Scanning Electron Microscope) cross-section micrograph of the electrodes. The cell's chemistry is mainly based on cobalt oxide-based cathode and graphite-based anode, as confirmed by Energy-dispersive X-ray spectroscopy (EDX) analysis. The electroactive materials' thickness and the relatively thin current collectors provide an optimum condition to maximize the energy density without compromising the power handling of this battery.
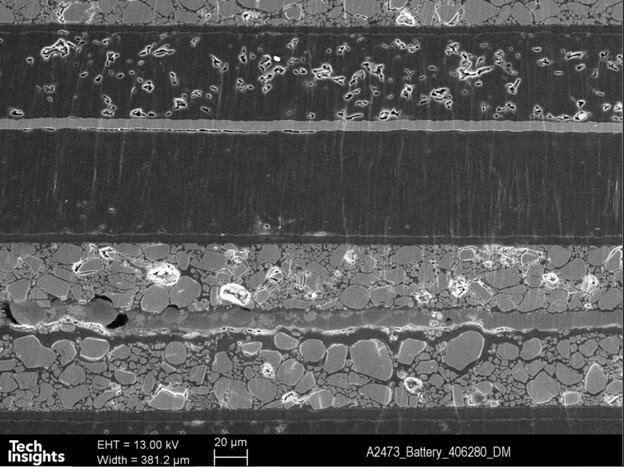
Figure 7: Detailed cell stack-up –SEM Cross Section of Apple watch series 7 (41 mm)’s battery.
The separator of this battery is found to be an organic polymer coated with a ceramic layer on both sides, Desay used a specific ceramic to enhance the battery’s safety in a thermal runaway condition.
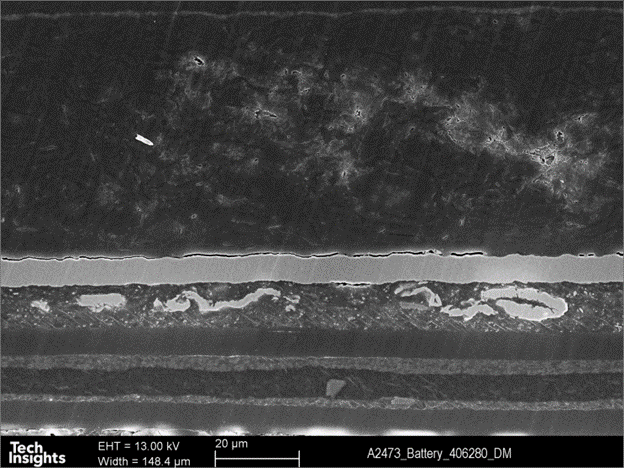
Figure 8: SEM Cross-Section image of the metal pouch of Apple watch series 7 (41 mm)’s battery.
Discussion on Formfactor
One of the goals of the metal can formfactor is that it aims to reduce battery size. This leads to the question, what if the jellyroll (Figure 6) was encased in a conventional polymer casing? Polymer casings are usually thicker, ~100 μm increasing the overall thickness of the battery by 200 μm (0.2 mm) for width, length, and height. Secondly, in the case of a polymer pouch, it is not possible to connect the entire body of the pouch and other electronic components of the device to a common ground, losing an additional safety feature. Additionally, swelling of the battery cannot be controlled, since this design lets the metal can swell in a controlled way as is shown in our report [2].
There might also be an additional benefit which has not been explicitly described in the patent. This battery may have a longer cyclic life since the compression of the can may prevent the delamination of the battery layers during cycling.
Summary:
In this blog, we reviewed a new design of lithium-ion batteries patented by Apple Inc and manufactured by Desay's for the Apple smartwatch series 7 – 41 mm. In this design, the jellyroll of the battery is encased in a stainless-steel can casing and the entire case is connected to the negative electrode of the battery. The cell cathode is composed of CoO2, and the anode is composed of graphite. The electrodes are separated by a polymer coated with ceramic on both sides. Compared to the typical pouch cell, this new embodiment offers several advantages, as summarized below.
This new design does not increase the energy density of the battery; however, it reduces the spacing needed between the battery and other components, making it possible to build smaller consumer electronics while maintaining battery safety. In this design, the electrode assembly is electrically coupled with the housing to form a common ground to allow other components to contact the battery housing without causing a short circuit or corroding the parts. Additionally, in the case of swelling, this new pouch design lets the pouch expand so that the overall size of the battery remains constant.
Additional detail and analysis of this battery can be found in the report "Desay's A2663 Metal Can Battery (Apple Watch Series 7 - 41mm) Essentials" which is part of the TechInsights Rechargeable Battery Essentials subscription. [3].
References
- Yuan, X., H. Liu, and J. Zhang. "Lithium-ion batteries: advanced materials and technologies. CRC." (2011).
- Pelletier, David M., et al. "Metal can battery." U.S. Patent Application No. 16/883,959.
- Report










